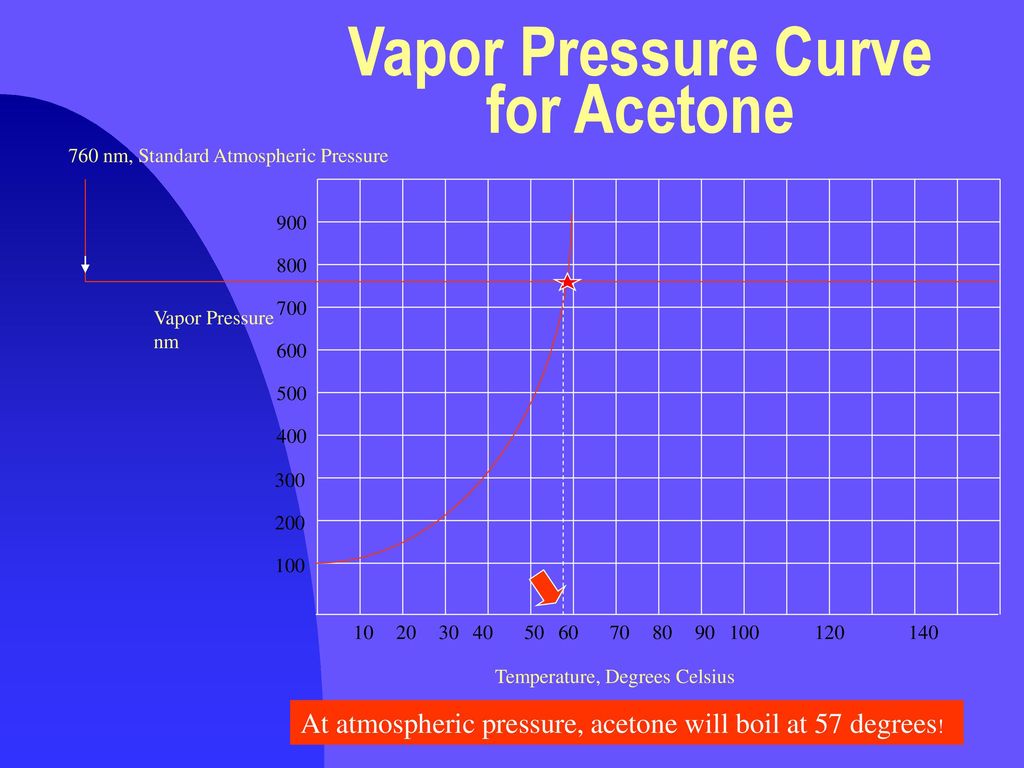
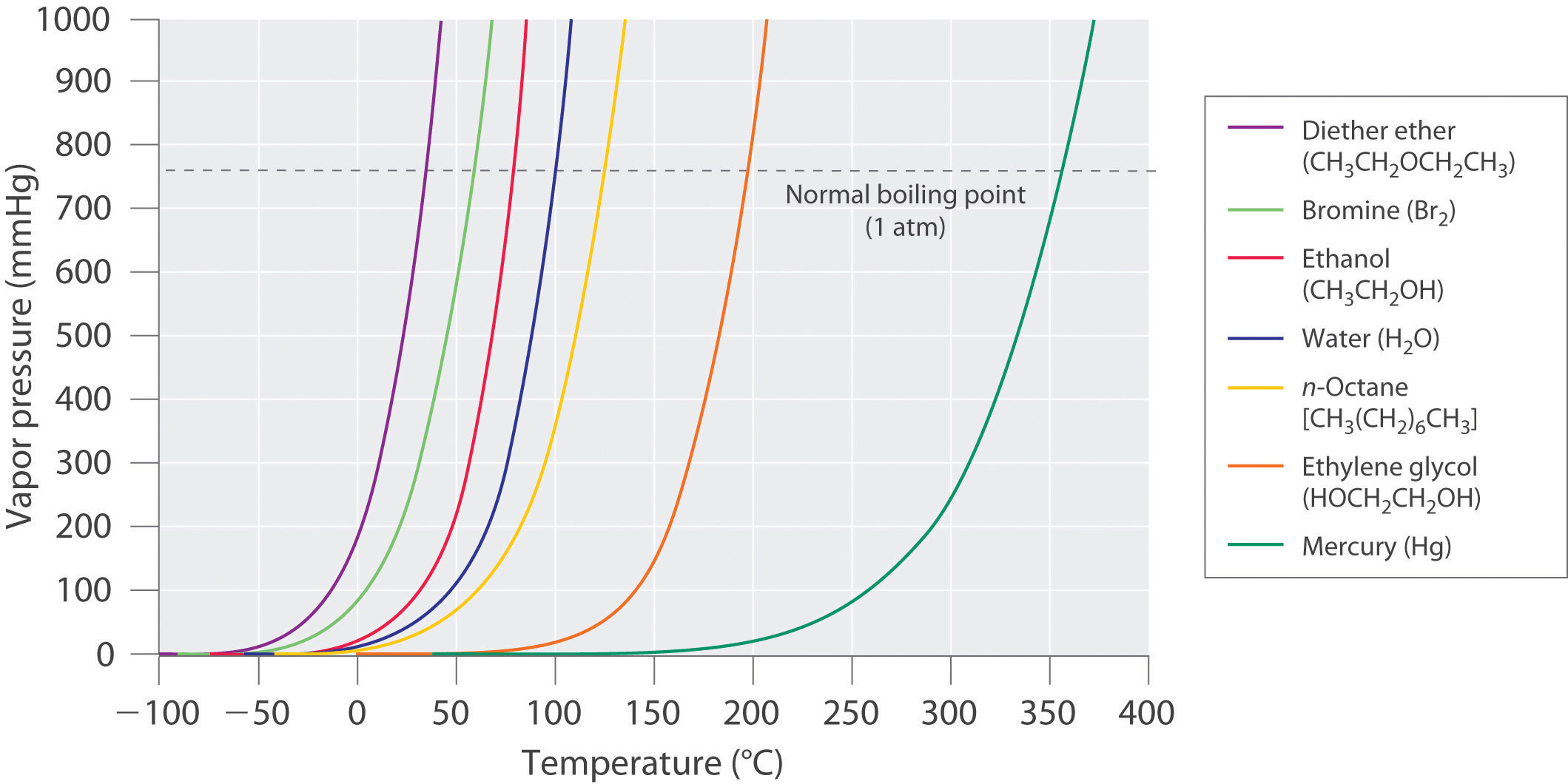
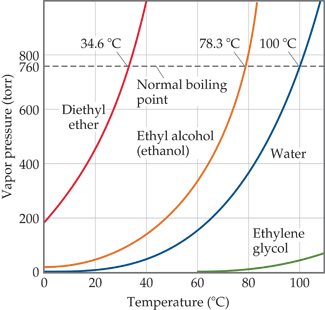
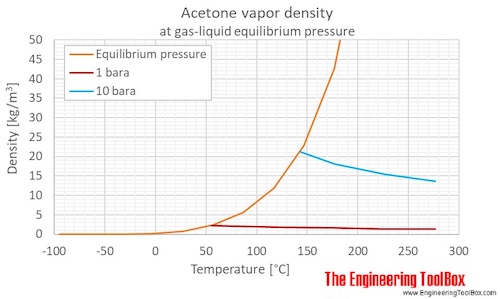
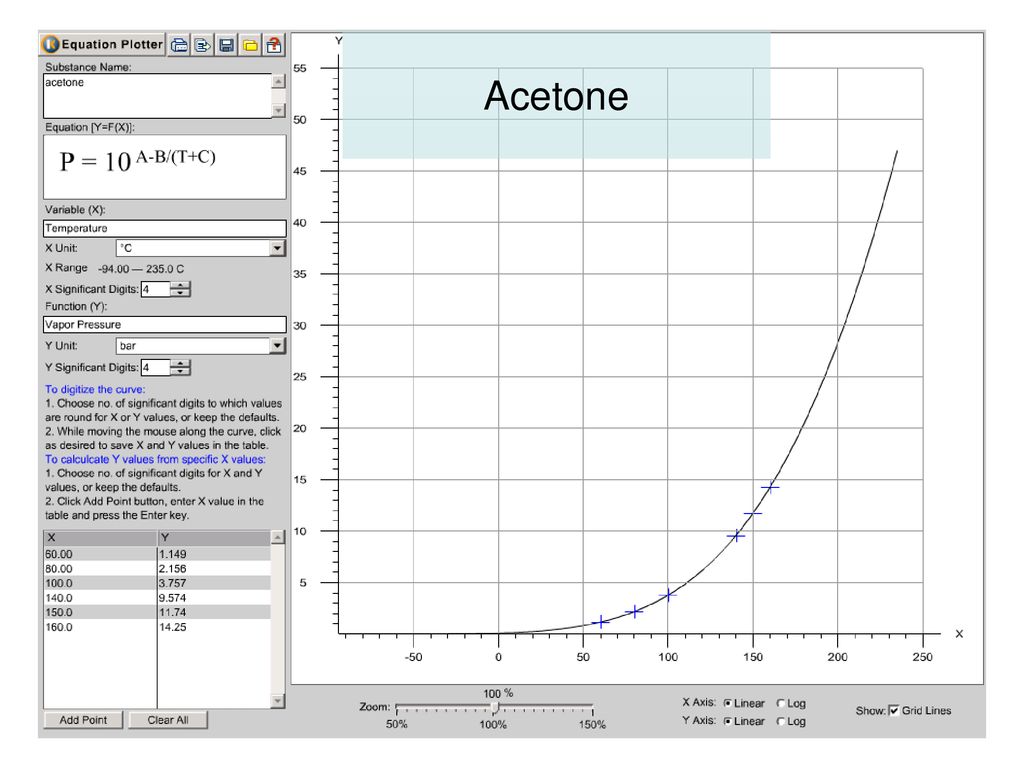
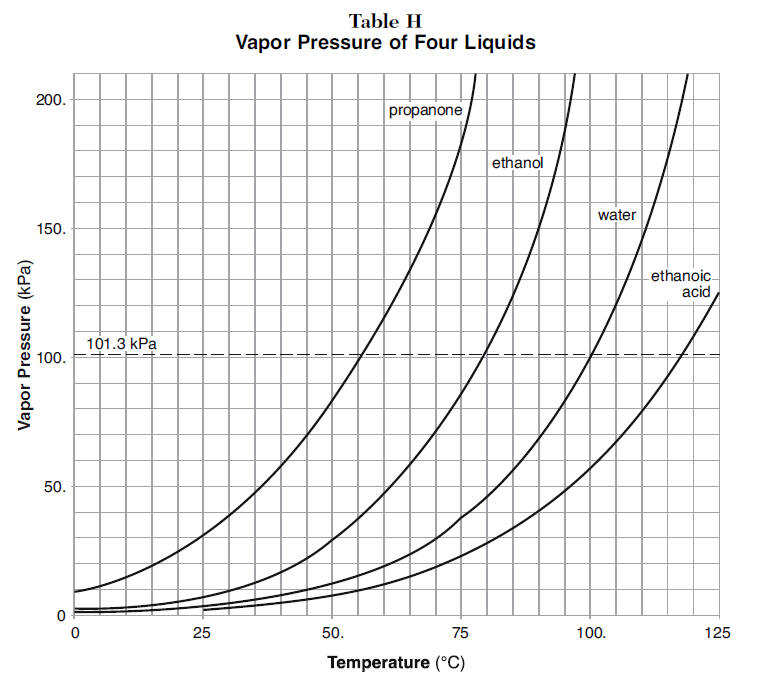
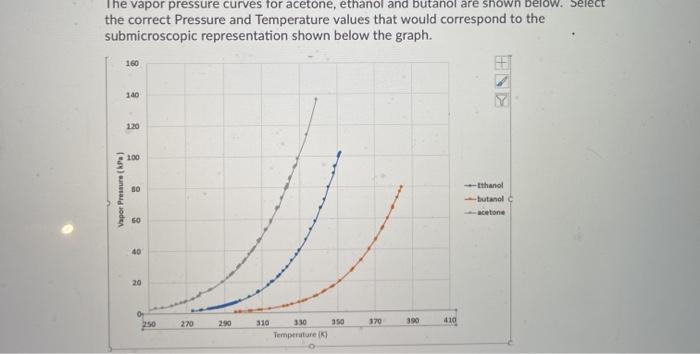
Use the following vapor pressure diagram to estimate the partial pressure of acetone. | Homework.Study.com
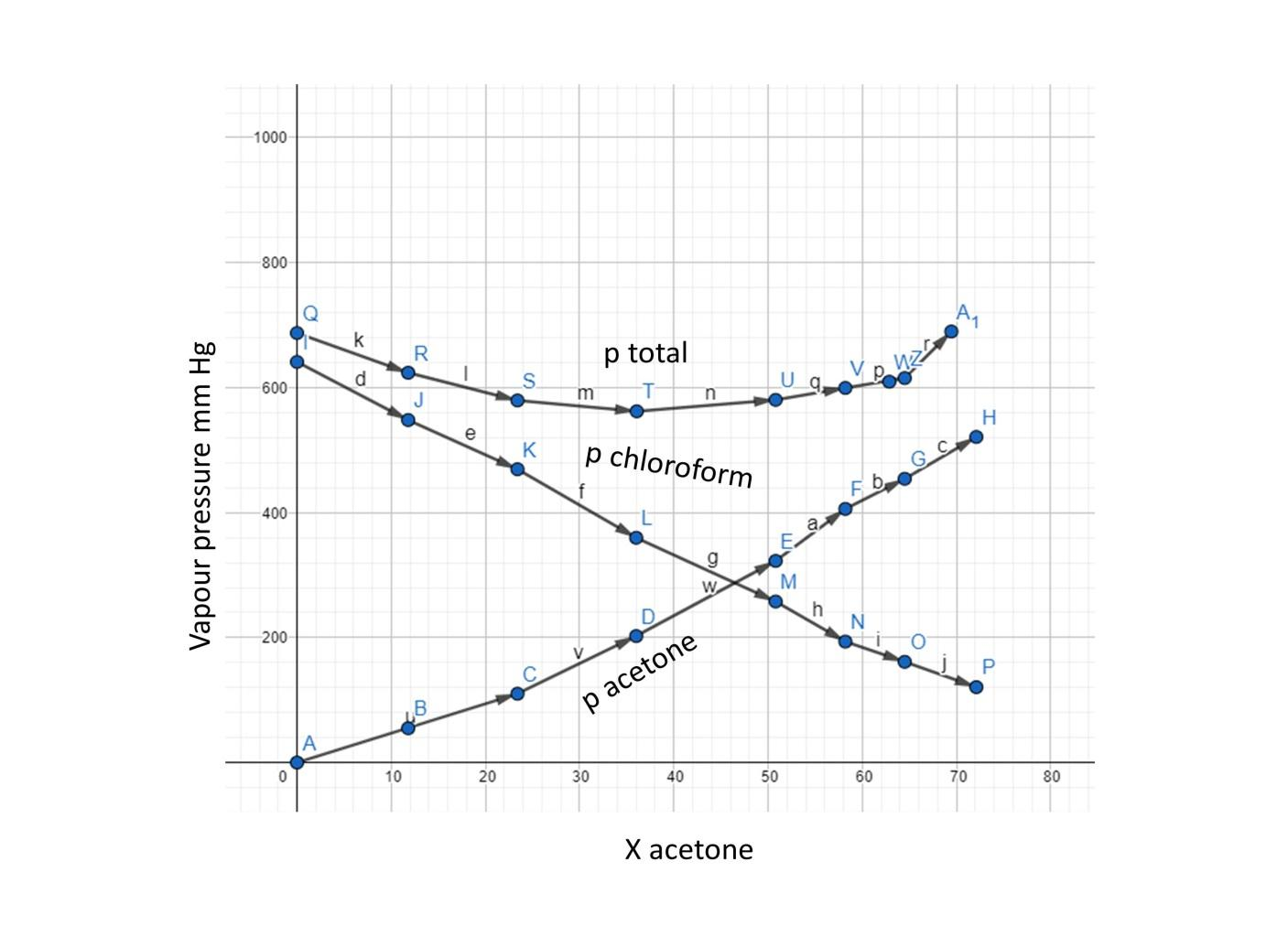
Vapour pressure of pure acetone and chloroform at $328\\,K$ are $741.8mmHg$ and $632.8mmHg$ respectively. Assuming that they form an ideal solution over the entire range of composition. Plot ${P_{total}},{P_{chloroform}}$ and ${P_{acetone}}$ as

Vapour pressure of pure acetone and chloroform at 328 K are 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot

The saturation vapor pressure of the benzene/acetone mixture (C 6 H 6 +... | Download Scientific Diagram